Addgene Website Feedback
Please note: Your browser does not support the features used on Addgene's website. You may not be able to create an account or request plasmids through this website until you upgrade your browser. Learn more
Please note: Your browser does not fully support some of the features used on Addgene's website. If you run into any problems registering, depositing, or ordering please contact us at [email protected] . Learn more

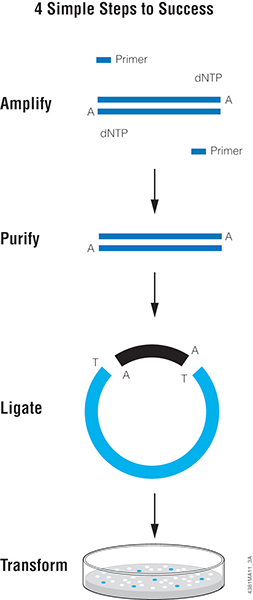
DNA Ligation
You may also like....
- Plasmid Cloning by Restriction Enzyme Digest
- Restriction Digest of Plasmid DNA
- Bacterial Transformation
Background Information
The final step in the construction of a recombinant plasmid is connecting the insert DNA (gene or fragment of interest) into a compatibly digested vector backbone. This is accomplished by covalently connecting the sugar backbone of the two DNA fragments. This reaction, called ligation, is performed by the T4 DNA ligase enzyme. The DNA ligase catalyzes the formation of covalent phosphodiester linkages, which permanently join the nucleotides together. After ligation, the insert DNA is physically attached to the backbone and the complete plasmid can be transformed into bacterial cells for propagation.
The majority of ligation reactions involve DNA fragments that have been generated by restriction enzyme digestion . Most restriction enzymes digest DNA asymmetrically across their recognition sequence, which results in a single stranded overhang on the digested end of the DNA fragment. The overhangs, called "sticky ends", are what allow the vector and insert to bind to each other. When the sticky ends are compatible, meaning that the overhanging base pairs on the vector and insert are complementary, the two pieces of DNA connect and ultimately are fused by the ligation reaction.
The example below depicts the ligation of two sticky ends that were generated by EcoRI digestion:
Usually, scientists select two different enzymes for adding an insert into a vector (one enzyme on the 5' end and a different enzyme on the 3' end). This ensures that the insert will be added in the correct orientation and prevents the vector from ligating to itself during the ligation process. If the sticky ends on either side of the vector are compatible with each other, the vector is much more likely to ligate to itself rather than to the desired insert. If you are in this situation, it is important to treat the digested vector backbone with a phosphatase before performing the ligation reaction (phosphatase removes the 5' phosphate and therefore prevents the ligase from being able to fuse the two ends of the vector together).
Protocol: Standard Insert + Vector DNA Ligation
Before setting up the ligation reaction itself, it is important to determine the amount of cut insert and vector to use for the ligation reaction. The volume of vector DNA and insert DNA used in the ligation will vary depending on the size of each and their concentration. However, for most standard cloning (where the insert is smaller than the vector) a 3 insert : 1 vector molar ratio will work just fine. We recommend around 100ng of total DNA in a standard ligation reaction. Use a (Link opens in a new window) ligation calculator to easily quantify how much vector and insert DNA to use.
- Ligase Buffer (1μL/10μL reaction for 10X buffer, and 2μL/10μL reaction for 5X buffer)
- 0.5-1μL T4 DNA Ligase
- H 2 O to a total of 10μL
- If the DNA concentrations are low such that you cannot get all 100ng of DNA, buffer and ligase into a 10μL reaction, scale the reaction size as necessary - being sure to increase the amount of buffer proportionally. 1μL of ligase should be sufficient for larger ligation reactions.
- Because ligase buffer contains ATP, which degrades upon freeze/thaw cycles, it is a good idea to take a fresh tube, thaw it one time and aliquot individual tubes of 5, 10 or 20μL for storage at -20°C. Whenever you need to set up ligations in the future you can thaw a new tube that you know has only been thawed once before.
- Always do controls. See Tips and FAQ below for details.
- Try different vector to insert ratios to optimize the ligation reaction. See Tips and FAQ below for details on optimization.
Note: For many ligation reactions, especially if using "high concentration" ligase, 5min at room temperature is enough. For trickier ligations (such as ligation of annealed oligos) the efficiency of ligation can be improved by incubation at 37°C.
- Proceed with bacterial transformation .
Tips and FAQ
Do controls.
When doing ligations you should ALWAYS do a vector alone + ligase control. This will allow you to verify that the vector was completely digested and if phosphatase treated, that the phosphatase treatment worked. This control should, in principle, be free of colonies, but the reality is that it will have some amount of background. What you want to see is that your vector + insert ligation has many more colonies than your vector alone ligation.
Additional controls are encouraged, but may only be required for troubleshooting failed ligations. The following table indicates the various controls:
| Control | Ligase | Interpretation |
|---|---|---|
| Uncut vector | - | Checks viability of competent cells and verifies the antibiotic resistance of the plasmid |
| Cut vector | - | Background due to uncut vector |
| Cut vector | + | Background due to vector re-circularization - most useful for phosphatase treated vector |
| Insert or water | + | Any colonies indicate contamination of intact plasmid in ligation or transformation reagents |
Optimizing the Vector:Insert Ratio
Although a 3:1 insert to vector ratio is usually sufficient, you can optimize the amount of insert and vector to improve ligation efficiency in situations where the 3:1 ratio is not working or when doing more complicated cloning. While 3:1 will get you in the ballpark for average size genes and vectors, this ratio is really meant to refer to the molarity of DNA ends available for ligation. Simply put, there are only two ends on any given piece of DNA no matter how long it is, and therefore we need to adjust the amount of DNA used in a ligation based on the length of the DNA to get a proper ratio of 3 available insert ends for every available vector end. (Link opens in a new window) Ligation calculators are easily found on the web. Just enter the concentration, lengths of your insert and vector, and what ratio you want, and it will tell you exactly how much of each to use.
- Reference Page
Search Thermo Fisher Scientific
- Quick Order
- Check Order Status
- Custom Products & Projects
- Instrument Management
- Home ›
- Brands ›
- Thermo Scientific ›
- Molecular Biology ›
- Molecular Biology Resource Library ›
- Molecular Biology Spotlight Articles ›
- Optimize Your DNA Ligation with 7 Must-Have Tips
Optimize Your DNA Ligations
- ‹ Molecular Biology Resource Library
- Popular resources
- Molecular Biology Education
- Webinars On-Demand
- Selection guides and tools
- T m Calculator
- DNA Ladders Selection Guide
- PCR Enzymes
- PCR and qPCR Plastic Consumables
- Reverse Transcriptase
- Restriction Enzyme
In this article, we share seven must-have tips for your ligation reactions:
- Consider your cloning strategy
- Check the ends of your DNA inserts
- Set up ideal reaction conditions
- Avoid inhibitors
- Visualize your ligation reactions on a gel
- Run controls
- Check to make sure your ligase is active
Molecular cloning is the process used for taking recombinant DNA (referred to as an insert) and placing it into a DNA vector (i.e., plasmid) where it can be replicated and expressed. This process involves multiple steps (such as copying the DNA, cutting out the gene of interest, and pasting the gene into the DNA vector). The final step, ligation (aka the pasting step) is used to seal the insert into the vector. Ligation works by using a phosphodiester bond to connect the sugar backbone of the double-stranded DNA insert with the sugar backbone of the double-stranded DNA vector. This is typically done by using T4 DNA ligase .
T4 DNA ligase is an enzyme that helps create the formation of a phosphodiester bond between the 3'-hydroxyl end of a double-stranded DNA fragment and the 5'-phosphate end of the same or another DNA fragment (Figure 1) . T4 DNA ligase can catalyze a reaction between blunt-end (no overhangs) or sticky end (3' or 5' complementary single-stranded overhangs) DNA fragments. T4 DNA ligase activity requires Mg2+ and ATP to work, and requires 5'-phosphorylation of one or both fragments.
Figure 1. T4 DNA ligase reaction mechanism.
There are many variables that are necessary to obtain maximum ligation efficiency and accuracy during cloning. As such, the ligation step can fail for numerous reasons, including:
- Problems related to the ligase enzyme itself (concentration, enzyme stability)
- Issues that occur before the addition of T4 DNA ligase (for example, presence of inhibitors including salts, EDTA, proteins, phenol, ethanol, and dATP)
- Everything else (molar insert to vector ratio, temperature, buffer composition, etc.)
To help your cloning experiment move forward, here are seven tips to help when you encounter DNA ligation failures:
Tip 1: Consider your cloning strategy
Different cloning strategies can be used depending on the type of ends on the DNA fragments you are using ( Figure 2 ).
- Sticky ends must be compatible
- Cloning is directional
- Insert-vector ligation is efficient
- Vector self-ligation is low
- Recognition sites of ligated restriction enzymes are intact
- Recognition sites of original restriction enzymes (e.g., Xhol and SaII) may be destroyed after ligation
- Directional cloning is maintained
- Ligation of the blunt ends may be less efficient
- Ends are compatible
- End sequences are modified
- Directional cloning is lost
- Ligation may be less efficient
- Vector self-ligation is high
The first three panels in Figure 2 illustrate strategies for cloning fragments with distinct sticky ends. These fragments are created when restriction enzymes cut in different places within the double-stranded DNA, resulting in overhangs (unpaired nucleotides). These “sticky ends” can be very helpful and are something that should be considered first when designing your cloning strategy. For example, if you want to clone your DNA insert so that it is read in a specific direction or orientation (also known as directional cloning), sticky end ligation is the method of choice since the created overhangs will only ligate in a specific orientation. Another reason for using sticky ends is to increase your ligation efficiency. When compared with the alternative (blunt-end ligation), sticky end ligation is more efficient due to the compatible overhangs that assist with the ligase reaction.
However, it is not always possible to use restriction enzymes that cut in different places to create sticky ends. For those cases, you would choose a blunt-end cloning strategy to ligate your DNA insert into your vector as illustrated in the fourth panel of Figure 2 . For this approach, you can either choose a restriction enzyme that will generate blunt ends, or you can generate sticky ends and remove the overhangs by using an end repair kit . Since blunt-end ligation is less efficient than sticky end ligation, this approach will require additional optimization and planning. Using a higher DNA insert to vector ratio is recommended to help ensure ligation of your DNA insert into your vector while preventing vector re-circulization (ligation of your vector without the DNA insert). To further help prevent vector re-circulization, treating the vector with DNA phosphatase helps to remove the 5’-end phosphate groups from the vector before the ligation step.
Tip 2: Check the ends of your DNA inserts
If the DNA inserts you are ligating have blunt ends, the inserts must be 5'-phosphorylated at both ends in order for ligation to occur. If the DNA insert is generated from restriction enzyme digestion, the 5'-phosphate group is already present.
However, if your DNA insert is a PCR product created with a proofreading DNA polymerase , your DNA insert will not have a 5’-phosphate group. Therefore, a phosphate group must be added using T4 polynucleotide kinase (T4PNK) .
When your DNA insert is a PCR product created with a Taq -like DNA polymerase, the resulting PCR product will have deoxyadenosine (dA) protruding ends since Taq DNA polymerases add a single dA to the 3´ ends of PCR products. DNA inserts that have dA ends can be ligated into vectors with complementary overhangs (this is a technique known as TA cloning). If, however, the vector you are using with TA cloning contains blunt ends, then your DNA insert also must be blunted (overhangs removed) before ligation.
If the DNA inserts you are ligating have sticky ends, they not only need the 5’-phosphates, but you will also need to make sure that the overhangs (sticky ends) on the inserts are complementary to your vector. If your overhangs are not complementary (ragged ends), your insert will not “paste” to your vector and your ligation will fail. Ragged ends can occur due to incomplete restriction enzyme digestion, elimination of your overhangs by a DNA polymerase that was not removed during purification of your insert, or by contaminating nucleases that might be present in the enzymes used to create the ends of your DNA insert. The best way to determine the problem is by running a set of controls with your experiment ( see Tip 6 below ).
Tip 3: Set up ideal reaction conditions
In order for the ligation of your DNA insert and vector to work, it is critical to ensure that proper reaction conditions have been set. The ideal ligation reaction conditions are dependent on many factors, including the concentration of reaction components, reaction temperatures, and reaction times. If any of these factors are not optimized, the DNA ends of the insert and vector may fail to anneal frequently enough for ligase to seal the fragments together.
Reaction components
Here’s a recommended ligation reaction protocol that can serve as a starting point for your optimization:
| Component | Amount (sticky end) | Amount (blunt end) |
|---|---|---|
| Vector | 20–100 ng | 20–100 ng |
| Insert ( ) | x ng | x ng |
| 10x ligation buffer* | 2 µL | 2 µL |
| 50% PEG 4000 solution (blunt ends**) | 2 µL | 2 µL |
| T4 DNA ligase (sticky ends) | 1.0–1.5 Weiss Units | |
| T4 DNA ligase (blunt ends) | 1.5–5.0 Weiss Units | |
| Water, nuclease-free | to 20 µL | to 20 µL |
| Incubation time: Ten minutes to one hour at 22°C | ||
* Ligation buffer includes ATP and DTT (a reducing agent), both of which degrade after multiple freeze-thaw cycles or extended incubations. In addition, DTT is prone to degradation during multiple exposures to oxygen, which also occurs through multiple freeze-thaw cycles. Since successful ligation is partly dependent on the correct concentrations of ATP and DTT, it is recommended to freeze ligation buffer in small single-use aliquots to prevent this freeze-thaw degradation. ** Blunt-end ligation is less efficient than sticky end ligation, so a higher concentration of ligase plus a crowding agent like polyethylene glycol (PEG) should be used for faster ligation.
Another optimization step is in the determination of the insert:vector ratio. The equation below can be used to calculate the even molar ratio in nanograms of insert DNA to vector DNA based on length:
| length of insert (bp) | x ng of vector = ng of insert needed for 1:1 insert:vector |
| length of vector (bp) |
To determine the best ratio of insert:vector to use for cloning, you may have to try different ratios ranging from 1:1 to 15:1, but a 3:1 ratio is a good place to start. For blunt-end ligation, be sure to adjust the insert:vector ratio and increase to 10:1 to optimize your result.
Reaction temperatures
In general, T4 DNA ligase is a temperature-sensitive enzyme. Therefore, reaction efficiency and ligase activity decrease dramatically when the temperature is raised higher than 37°C. The ligation reaction should be incubated at room temperature. To increase reaction rates, the temperature can be cycled between the optimal temperature for the T4 DNA Ligase and the annealing temperature of the overhangs.
Reaction times
In general, prolonged incubation times are not necessary since ligase is a very efficient enzyme. Typically, the ligation should work at room temperature (~22°C) with reaction times ranging from ten minutes to one hour. In rare cases, such as when working with very long DNA fragments, overnight incubations of ligation reactions are necessary.
Tip 4: Avoid inhibitors
Several compounds can inhibit ligation reactions, including salts (like sodium chloride, potassium chloride, ammonium), EDTA, proteins, phenol, ethanol, and dATP. Since these inhibitors can be present in abundance, steps must be taken to either avoid concentrating the inhibitors or removing them from the reaction.
It can be tempting to concentrate the amount of vector and insert in your reaction by reducing the final volume (e.g., 10 µL versus 20 µL) of your ligation. However, while this will increase the concentration of DNA present, it may also increase the concentration of inhibitors present in the reaction. In general, a final ligation reaction volume of 20 μL is recommended since this volume includes ~10 μL of pure water (which is added to the reaction) to dilute any inhibitors that are present. In addition, by keeping your glycerol at less than 5% of the final reaction volume, this helps prevent excess glycogen from acting as an inhibitor in the ligase reaction.
Another option to deal with inhibitors present in your reaction is to remove them through a DNA purification stop. For this approach, commercially available silica-based columns are the preferred method of choice since the silica particles will not carry over through purification into the ligation reaction. If you choose to use silica matrix particles instead of columns, be aware that residual particles that may carry over can bind the ligase, thereby inhibiting the ligation reaction. If silica matrix particles are your only option, you will need to perform a short centrifugation step prior to the ligation reaction.
NOTE: If electrocompetent cells are being used for transformation following ligation, an additional column purification step of the ligated DNA should be used to remove any salt contaminants that may be present and therefore cause damage to your cells during electroporation.
Tip 5: Visualize your ligation reactions on a gel
When your cloning experiment didn’t work, it can be difficult to determine what went wrong. Since the success of your ligation reaction is crucial for cloning success, it is preferred to check your ligation reaction to confirm the insertion of your DNA insert into the vector before moving to the next step in your cloning workflow.
The confirmation of ligation reactions can be monitored using agarose gel electrophoresis. To do so, we recommend running the following samples out on a gel:
- DNA before ligation reaction (e.g., unligated vector plus DNA insert)
- DNA after ligation reaction (e.g., ligated vector plus DNA insert)
Before running your samples on the agarose gel, mix with an SDS-containing loading dye and incubate at 65°C for ten minutes. Adding the SDS to your loading dye allows for disassociation of ligase from the DNA in the sample, which prevents smearing on the gel. After the ten-minute incubation, run the samples on your gel. If your reaction was successful, the sample containing the ligated products would migrate at a higher molecular weight range than the sample with the unligated products.
Figure 3 illustrates how ligation reaction products may resolve on an agarose gel:
Figure 3. Ligation reaction performed with T4 DNA ligase shown via agarose gel. Lane 1: Vector and insert before ligation. Lane 2: After ligation, loading dye without SDS. Lane 3: After ligation, loading dye with SDS. Lane M: Thermo Scientific GeneRuler DNA Ladder Mix (Cat. No. SM0331 ).
As shown in the gel, the ligation reaction appears:
Lane 1: The bright, clear bands represent the vector and DNA insert before ligation.
Lane 2: No SDS results in a smear in the lane as a result of the ligase that remains bound to the DNA.
Lane 3: With the addition of SDS, the ligase is no longer bound to the DNA, and clear higher molecular weight bands are seen, representing the ligation products in the reaction. In addition, the vector and DNA insert bands are diminished, indicated that the amount present has decreased.
Lane M: The size and approximate amount of ligation product, unligated vector and insert is determined by comparing bands to known size and amount of each band of a DNA ladder used as a marker.
Tip 6: Run controls
Controls are critical components of any experiment. The table below lists common ligation reaction controls that can help track the success of the ligation steps in your cloning workflow.
| Reaction setup | Purpose of the control and interpretation | Expected results |
|---|---|---|
| Uncut vector (No ligase) | ✓ Checks quality and efficiency of competent cells ✓ Verifies antibiotic selection | |
| Cut vector (No ligase) | ✓ Determines background from uncut vector ✓ Checks restriction digestion efficiency | |
| Cut vector + Phosphatase + Ligase | ✓ Reveals background from any recircularized vector ✓ Checks efficiency of DNA phosphatase treatment | |
| Insert or water + Ligase | ✓Checks for contamination of reagents, stock solutions, or pipettes |
Tip 7: Check to make sure your ligase is active
As mentioned previously, T4 DNA ligase is a temperature-sensitive ligase and is inactivated at higher temperatures. If your ligase was stored or shipped improperly, it could be denatured or lose some activity. Since your ligation reaction can fail from due to denatured or impaired ligase, you should always follow the manufacturer’s recommendations for enzyme storage conditions and usage.
If you think your ligase may have been denatured or inactivated, check the following:
- The expiration date on the vial
- The ligase activity by using a positive control
For example, the commercially available DNA Marker Lambda DNA/HindIII can be used as a positive control. When used in a ligation reaction with T4 DNA Ligase, the band pattern in the sample, when run on a gel, will show a single higher molecular weight band when the ligase is active. If the ligase is not active or is diminished, the original banding pattern of the Lambda DNA/HindIII marker will remain (multiple bands will show on the gel).
Summary of ligation tips
There are many reasons why ligation reactions can fail, with the most common arising from problems that occur before the addition of the T4 DNA ligase. When setting up or troubleshooting your ligation reactions, be sure to remember the tips listed below to help enable successful cloning results every time:
- Consider your cloning strategy
- Check the ends of your DNA inserts
- Avoid inhibitors
- Visualize your ligation reactions on a gel
- Run controls
- Check to make sure your ligase is active
To further improve your experiment, explore our extensive offering for ligation reactions in more detail:
- Thermo Scientific T4 DNA Ligase
- Rapid DNA Ligation Kit
- T4 polynucleotide kinase (T4PNK)
- FastAP Alkaline Phosphatase
- Fast DNA End Repair Kit
To learn more about different cloning strategies, read Common Cloning Applications and Strategies
Molecular Biology Resource Library
Access valuable support for standard molecular biology techniques from our library of webinars, videos, articles, and more.
Molecular Biology Web Tools
Utilize free online tools for primer analysis, reaction setup, biochemical conversions, and calculators.
Product selection guides
- DNA Ladders
- FastDigest Restriction Enzyme
- qPCR and PCR Plastics
- TOPO PCR Cloning
Molecular Biology Handbook
Optimize your experiments. Our latest edition is improved with more technical tips, educational tools, and guidance to select the right molecular biology products.
Molecular Biology Mini Catalog
Find the right products for your experiments. Our mini-catalog features the most popular Thermo Scientific molecular biology products.
Molecular Biology Product Discontinuations
Find an alternate to a discontinued molecular biology product.
Molecular Biology Services and Support
Email or call our Technical Application Scientists for additional questions regarding molecular biology products.
Unable to load all required components properly.
Please try reloading the page. If that fails, try clearing the browser cache for this site and then reload the page.
Quick Tips - How do I calculate how much DNA to add to a ligation reaction?

Share this post:
How do I calculate how much DNA I need to add to a ligation reaction?
So, for typical cloning applications we recommend 50 ng of the vector, which will be 25-30 fmoles depending on your vector size. For inserts of similar size to the vector, we recommended a 1:1 molar ratio of insert to vector. If your insert is smaller than the vector, say if you’re trying to ligate a 1kb insert into a 3kb vector, you’ll need a higher ratio, in this case about a 3:1 molar ratio of insert to vector. If your inserts are very small, even higher ratios may be needed, sometimes as high as 20:1.
You can calculate the amount of DNA you need for your specific reaction using the NEBiocalculator on NEB.com.
And remember, if you have any addition questions, you can always contact us via email at [email protected].
Related Videos

Choose your country
North america, asia-pacific.
If you don't see your country above, please visit our international site
You have been idle for more than 20 minutes, for your security you have been logged out. Please sign back in to continue your session.
Your profile has been mapped to an Institution, please sign back for your profile updates to be completed.
To save your cart and view previous orders, sign in to your NEB account. Adding products to your cart without being signed in will result in a loss of your cart when you do sign in or leave the site.
DNA ligation
(KA Longo, 1/01)
Quantitate your cut vector DNA and insert using UV spectroscopy. If your vector has been cut with a single enzyme, you'll want to treat it with Calf Intestinal Alkaline Phosphatase (CIP) first.
Set up the following reaction:
* The amount of insert DNA used will depend on the relative size of the insert compared to the size of the vector. A molar ratio between insert and vector should be calculated.
Typically, the insert/vector ratio for sticky-end ligations ranges between 1:1 and 3:1. For blunt-ended ligations, the insert/vector ratio should be at least 10:1.
The number of moles for vector and insert can be calculated as follows:
------------------------------------------
[(# of base pairs DNA) x (660 daltons/bp)]
You can use the following equality to figure out the amount of insert DNA needed for a known amount of vector in a blunt-end ligation:
(g vector DNA) (g insert DNA)
----------------------------------------- x 10 = -----------------------------------------
[(# of bp vector DNA) x (660 daltons/bp)] [(# of bp insert DNA) x (660 daltons/bp)]
10 x (g vector DNA) x (# of bp insert DNA)
g insert DNA = ----------------------------------------------------------
(# of bp vector DNA)
Note that any desired ratio can be substituted for the number 10 in the above equation, depending on the type of ligation you are performing.
Our website does not fully support your browser.
For the best browsing experience, please upgrade to a modern web browser.
Promega's Cookie Policy
We use cookies and similar technologies to make our website work, run analytics, improve our website, and show you personalized content and advertising. Some of these cookies are essential for our website to work. For others, we won’t set them unless you accept them. To find out more about cookies and how to manage cookies, read our Cookie Policy .
If you are located in the EEA European Economic Area , the United Kingdom, or Switzerland, you can change your settings at any time by clicking Manage Cookies in the footer of our website.
It looks like you are having trouble logging in, please try our dedicated login page.
Thank you for verifying your email address.
There was an issue verifying your email address. Please try again or contact Customer Service.
Contact Customer Service
Biomath Calculators
Note: When entering decimal values in the Biomath calculators, please use a decimal point “.” rather than “,” as these calculators use decimal points for input/output of calculations.
DNA Conversions
dsDNA: µg to pmol dsDNA: pmol to µg ssDNA: µg/ml to pmol/µl ssDNA: pmol/µl to µg/ml Linear DNA: µg to pmol of Ends Ligations: Molar Ratio of Insert:Vector Nucleic Acid: OD 260 to µg/ml
Protein Conversions
Molar Conversions Coding Capacity of DNA
Other Calculators
Temperature Conversion
T m for Oligos
dsDNA: µg to pmol
Related nucleic acid analysis products and resources.
- Nucleic Acid Analysis
- Cloning and DNA Markers
- Nucleic Acid Extraction
Related Groups
- Cloning Vectors and Kits
- Molecular Biology Enzymes and Reagents
- PCR Cloning
- Taq Polymerase and Endpoint PCR

Guides and Selectors
- DNA Purification
- PCR Amplification
- How to Use Restriction Enzymes: A Resource Guide
Top Products
- T4 DNA Ligase
- GoTaq® G2 DNA Polymerase
- pGEM®-T Easy Vector Systems
Pacific Asia
Manage cookies.
Necessary Cookies
We use these cookies to ensure our site functions securely and properly; they are necessary for our services to function and cannot be switched off in our systems. They are usually only set in response to actions made by you which amount to a request for services, such as logging in, using a shopping cart or filling in forms. You can set your browser to block or alert you about these cookies, but some parts of our services will not work without them. Like the other cookies we use, strictly necessary cookies may be either first-party cookies or third - party cookies.
Allow Preference Cookies
We use these cookies to remember your settings and preferences. For example, we may use these cookies to remember your language preferences.
Allow Performance/Statistics Cookies
We use these cookies to collect information about how you interact with our services and to help us measure and improve them. For example, we may use these cookies to determine if you have interacted with a certain page.
Allow Marketing Cookies
We and our advertising partners use these cookies to deliver advertisements, to make them more relevant and meaningful to you, and to track the efficiency of our advertising campaigns, both on our services and on other websites and social media.
Your Privacy Choices
Our website supports your privacy choices by allowing users in select regions to opt out of specific types of sharing/sales/use of cookies and technologies for Targeted Advertising. Visit our Privacy Policy and Cookie Policy pages to learn more about these topics.
Targeted Advertising
Deselect this checkbox to disable cookies related to targeted advertising in your Promega experience. These advertising cookies will no longer be set for this browser and device.
Note: These settings are based on necessary cookies that are placed on your device, and if those cookies are cleared or you access the website from another browser or device, advertising cookies might be reset by default in the future, from which you can opt-out.
Promega Connections
Thoughts, tech tips and news about science, cloning with pgem®-t vectors: ligation.
One of the easiest methods for cloning blunt-ended DNA fragments including PCR products is T-vector cloning, such as with pGEM®-T or pGEM®-T Easy Vector Systems . This method takes advantage of the “A” overhang added by a PCR enzyme like Taq DNA Polymerase . T vectors are linearized plasmids that have been treated to add 3′ T overhangs to match the A overhangs of the insert. The insert is directly ligated to the T-tailed plasmid vector with T4 DNA ligase . The insert can then be easily transferred from the T vector to other plasmids using the restriction sites present in the multiple cloning region of the T vector.
Proofreading polymerases like Pfu do not add “A” overhangs so PCR products generated with these polymerases are blunt-ended. In a previous blog , we discussed a simple method for adding an A-tail to any blunt-ended DNA fragment to enable T-vector cloning. Below, we think about the next step: Ligation.
Ligation Preparation
You have blunt-ended your DNA insert of interest (from PCR, restriction enzyme digestion, or even sheared and blunted genomic DNA), made sure the fragment is A tailed and are ready to clone into a T vector (e.g., pGEM ® -T Easy Vector ). The next step is as simple as mixing a few microliters of your purified product with the cloning vector in the presence of DNA ligase , buffer and ATP.
Estimate the concentration of the prepared insert DNA by spectrophotometric method or compare the staining intensity of your PCR product with that of DNA molecular weight standard of similar size and known concentration on an agarose gel. If the vector DNA concentration is unknown, estimate the vector concentration by the same method you chose for the insert.
It’s a good idea to set up various vector:insert DNA ratios to determine the optimal ratio for a particular vector and insert. Keep in mind, the vector:insert ratio will change depending on the size of the insert. In most cases, a 1:1 or 1:3 molar ratio of vector:insert works well, but you may want to consider 1:5, 5:1 and even a 10:1 ratio.
The equation for calculation of the amount of insert required at a specific molar ratio of vector:insert is: [(ng of vector × kb size of insert) ÷ kb size of vector] × (molar amount of insert ÷ molar amount of vector) = ng of insert
Example: How much 500bp insert DNA needs to be added to 100ng of 3.0kb vector in a ligation reaction for a desired vector:insert ratio of 1:3? Answer: [(100ng vector × 0.5kb insert) ÷ 3.0kb vector] × (3 ÷ 1) = 50ng insert
If the math looks intimidating, don’t worry. Our BioMath Calculators are here to help!
If you’re interested in more details about how to set up a ligation, take a look at this helpful video:
Tips for T-Vector Cloning
Here are a few things to remember while you work:
- Nucleases may degrade the T overhangs on the vector. Make sure you use sterile, nuclease-free water in your ligation reactions.
- Use high-efficiency competent cells (≥1 × 10 8 cfu/μg DNA) for transformations. Ligation of fragments with a single-base overhang can be inefficient, so it’s essential to use cells with a high transformation efficiency to obtain a reasonable number of colonies.
- Limit exposure of your PCR product to shortwave UV light to avoid formation of pyrimidine dimers. Use a glass plate between the gel and UV source. If possible, only visualize the PCR product with a long-wave UV source, or use something like Diamond® Nucleic Acid Dye that allows you to visualize your DNA without the use of UV.
For more information on cloning, consult our Subcloning Technology Guide .
Are you an student or early-career scientist looking for troubleshooting and how-to resources? Or, do you supervise entry-level lab members? Check out our Student Resource Center for more helpful articles, videos and tools!
Related Posts
- Latest Posts

Leah Cronan
Latest posts by leah cronan ( see all ).
- Mass Spec for Glycosylation Analysis of SARS-CoV-2 Proteins Implicated in Host-Cell Entry - November 10, 2020
- Proteomics from a Different Point of View: Introducing ProAlanase, the Newest Mass-Spec Grade Protease from Promega - August 7, 2020
- Go fISH! Using in situ Hybridization to Search for Expression of a SARS-CoV-2 Viral Entry Protein - July 10, 2020
Share this:
if the vector is in linear form then how is it possible for it to produce blue colonies without a insert, the alpha fragment is disrupted if it is in a linear form.
Due to incomplete digestion or re-ligation, there will be a few circular plasmids. That’s why you need to estimate the background colonies.
Leave a Reply Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-advertisement | 1 year | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Advertisement". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| gdpr_status | 6 months 2 days | This cookie is set by the provider Media.net. This cookie is used to check the status whether the user has accepted the cookie consent box. It also helps in not showing the cookie consent box upon re-entry to the website. |
| lang | This cookie is used to store the language preferences of a user to serve up content in that stored language the next time user visit the website. | |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| SC_ANALYTICS_GLOBAL_COOKIE | 10 years | This cookie is associated with Sitecore content and personalization. This cookie is used to identify the repeat visit from a single user. Sitecore will send a persistent session cookie to the web client. |
| vuid | 2 years | This domain of this cookie is owned by Vimeo. This cookie is used by vimeo to collect tracking information. It sets a unique ID to embed videos to the website. |
| WMF-Last-Access | 1 month 18 hours 24 minutes | This cookie is used to calculate unique devices accessing the website. |
| _ga | 2 years | This cookie is installed by Google Analytics. The cookie is used to calculate visitor, session, campaign data and keep track of site usage for the site's analytics report. The cookies store information anonymously and assign a randomly generated number to identify unique visitors. |
| _gid | 1 day | This cookie is installed by Google Analytics. The cookie is used to store information of how visitors use a website and helps in creating an analytics report of how the website is doing. The data collected including the number visitors, the source where they have come from, and the pages visted in an anonymous form. |
| Cookie | Duration | Description |
|---|---|---|
| IDE | 1 year 24 days | Used by Google DoubleClick and stores information about how the user uses the website and any other advertisement before visiting the website. This is used to present users with ads that are relevant to them according to the user profile. |
| test_cookie | 15 minutes | This cookie is set by doubleclick.net. The purpose of the cookie is to determine if the user's browser supports cookies. |
| VISITOR_INFO1_LIVE | 5 months 27 days | This cookie is set by Youtube. Used to track the information of the embedded YouTube videos on a website. |
| Cookie | Duration | Description |
|---|---|---|
| BIGipServerwww.promega.com_sitecore | No description | |
| CanCheckOut | No description | |
| CommerceCustomerId | No description | |
| CONSENT | 16 years 7 months 15 days 6 hours 22 minutes | No description |
| cookies.js | session | No description |
| Country | 3 months | No description |
| CountrySelected | 3 months | No description |
| CustomerId | No description | |
| PreferredLanguage | 3 months | No description |
| PromegaCompno | 3 months | No description |
| PromegaCountry | 3 months | No description |
| RememberMe | 6 months | No description |
| SameSite | No description | |
| sc_ext_contact | 2 years | No description |
| sc_ext_session | session | No description |
| TS01ae363a | No description | |
| UID | 2 years | No description |
| website#lang | This cookie is used for storing the visitor language preferences. It heps in delivering localised language version. | |
| wp_api | past | No description |
| wp_api_sec | past | No description |
| _ga_WHZLGVEZ9X | 2 years | No description |
| Cookie | Duration | Description |
|---|---|---|
| YSC | session | This cookies is set by Youtube and is used to track the views of embedded videos. |
| _gat_UA-62336821-1 | 1 minute | This is a pattern type cookie set by Google Analytics, where the pattern element on the name contains the unique identity number of the account or website it relates to. It appears to be a variation of the _gat cookie which is used to limit the amount of data recorded by Google on high traffic volume websites. |
- Forum Index Home
- Live Discussion
Vector- Insert Ratio in Ligation - (Jan/12/2005 )
Hi, usually if you do a ligation it is recommended to use a vector- insert ratio 1:3 in general. I think this works great if the insert is smaller or maybe the same size as the vector. But what if the insert is about 3 times bigger than the vector. Is it then useful to try it the other way round: 3 times the vector to insert? Any ideas or experiences would be really great. I'm having trouble with one special cloning!!! Thanks to ya'll. Arwen76
the formular I have here at hand is from the promega portocols and applications guide "the source for discovery": (I abbreviated a bit) " -estimate the concentration of vector and insert by agarosegel-elpho or OD measurement -test various vector/insert ratios to find the optimal ratio -in most cases, a 1:1 or a 1:3 ratio work best formular: ((ng vector)x(kb size of insert))/(kb size of vector)) x (molar ratio of (insert/vector)) = (ng insert) example: 500bp insert to be ligated with 100ng of 3.0kb vector in a 3:1 ratio ((100ng vector)x(0.5 kb of insert))/(3.0 kb vector)) x (3/1)) = (50 ng insert) " hope that answers your question mike
the ratio is molar ratio, not volume. follow the formula given by jade falcon.
- Terms of Service
- Sponsorship
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 July 2024
Combination of error-prone PCR (epPCR) and Circular Polymerase Extension Cloning (CPEC) for improving the coverage of random mutagenesis libraries
- Natalia Ossa-Hernández ORCID: orcid.org/0000-0002-7785-7585 1 ,
- Luis Fernando Marins ORCID: orcid.org/0000-0002-7219-5295 2 &
- Daniela Volcan Almeida ORCID: orcid.org/0000-0001-5341-220X 3
Scientific Reports volume 14 , Article number: 15874 ( 2024 ) Cite this article
249 Accesses
Metrics details
- Expression systems
- Synthetic biology
Random mutagenesis, such as error-prone PCR (epPCR), is a technique capable of generating a wide variety of a single gene. However, epPCR can produce a large number of mutated gene variants, posing a challenge in ligating these mutated PCR products into plasmid vectors. Typically, the primers for mutagenic PCRs incorporate artificial restriction enzyme sites compatible with chosen plasmids. Products are cleaved and ligated to linearized plasmids, then recircularized by DNA ligase. However, this cut-and-paste method known as ligation-dependent process cloning (LDCP), has limited efficiency, as the loss of potential mutants is inevitable leading to a significant reduction in the library’s breadth. An alternative to LDCP is the circular polymerase extension cloning (CPEC) method. This technique involves a reaction where a high-fidelity DNA polymerase extends the overlapping regions between the insert and vector, forming a circular molecule. In this study, our objective was to compare the traditional cut-and-paste enzymatic method with CPEC in producing a variant library from the gene encoding the red fluorescent protein (DsRed2) obtained by epPCR. Our findings suggest that CPEC can accelerate the cloning process in gene library generation, enabling the acquisition of a greater number of gene variants compared to methods reliant on restriction enzymes.
Similar content being viewed by others
Golden Mutagenesis: An efficient multi-site-saturation mutagenesis approach by Golden Gate cloning with automated primer design
An improved overlap extension PCR for simultaneous multiple sites large fragments insertion, deletion and substitution
A high-efficiency method for site-directed mutagenesis of large plasmids based on large DNA fragment amplification and recombinational ligation
Introduction.
Proteins are biomolecules with significant potential for applications in research, the food and pharmaceutical industries 1 , and disease treatment 2 . Their amino acid sequences are determined by the information encoded in the genes. These sequences evolve due to spontaneous mutations, which are naturally selected by the environment or other factors. Numerous technologies have been developed to study protein evolution in the laboratory. These aim to accelerate mutational rates, generate a vast array of variants, and facilitate the selection of proteins with desired characteristics. Different alterations to the polymerase chain reaction (PCR) method have made both site-specific and random mutagenesis more accessible. This has improved enzyme stability across broader pH and temperature ranges and increased tolerance to various organic solvents. There are many specific methods for protein engineering, which can be broadly categorized into two main groups: those based on the rational design of protein modifications and combinatorial methods that introduce changes randomly (For review see 3 ).
Random mutagenesis, for example, is a technology capable of generating high diversity from a single gene that involves the creation of libraries consisting of large numbers of genetic variants, where each member can be isolated and individually assessed concerning the genotype–phenotype relationship 4 . Genetic variants can be obtained from methods such as error-prone PCR (epPCR). This last methodology, developed by Leung et al. 5 , uses a low-fidelity DNA polymerase that, under certain conditions, can introduce random mutations during PCR amplification of a target gene. The products of these mutagenic PCRs must then be linked to plasmid vectors and used to produce gene libraries. Although epPCR can generate an extremely large number of mutated gene, there is a limitation in ligating mutated PCR products into the plasmid vectors used to generate the libraries. The practical utility of any cloning method is based on its reliability, cost, or efficiency under optimal conditions. Methods that are easier to monitor and optimize are the most reliable 6 . The earliest cloning method used was Ligation-Dependent Cloning Process (LDCP) This method is used for cloning mutant fragments obtained by epPCR, for this the primers used are designed to include artificially specific restriction enzymes recognition sites that are compatible with the restriction sites present in the plasmid used. Thus, the PCR products must be cut with the chosen enzymes and then ligated to the plasmid previously linearized with the same enzymes. The cohesive termini will allow the recircularization of the vector by the action of a DNA ligase. This cut-and-paste process has limited efficacy and causes a significant reduction in the scope of the library since the loss of potential mutants is unavoidable.
Interesting alternatives to the LDCP to clone PCR products includes methodologies free of restriction enzymes and ligases 7 , 8 , 9 , and the the Circular Polymerase Extension Cloning (CPEC) method developed by Quan and Tian 10 . The last one consists of a reaction where a high-fidelity DNA polymerase extends the overlapping regions between the insert and vector to form a circular molecule. CPEC offers significant advantages in terms of simplicity, efficiency, and economy. It has even been suggested to produce gene libraries 11 . Compared to techniques reliant on restriction enzymes, the use of CPEC in creating gene libraries may accelerate the cloning process and produce a higher number of gene variations. In this case, our goal was to evaluate the effectiveness of CPEC vs the conventional cut-and-paste enzymatic technique for generating a variant library from the epPCR-obtained gene encoding the red fluorescent protein (DsRed2).
Material and methods
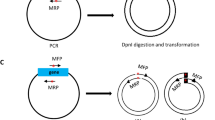
Step 1—obtaining the mutant insert by error-prone pcr and the control insert.
The DsRed2 gene was isolated using the plasmid pDsRed2 (Clontech, Cat. No. 632404, UniProt Q9U6Y8) as a template (Fig. 1 A). Error-prone PCR of the DsRed2 gene was performed using the GeneMorph® II Random Mutagenesis kit, following the manufacturer’s protocol The primers DsRed2-EcoRI-F and DsRed2-BamHI-R (Table 1 ) were used and the PCR conditions included one cycle at 94 °C for 2 min, followed by 30 cycles at 94 °C for 15 s, 68 °C for 30 s, and 72 °C for 60 s, with a final elongation step at 72 °C for 5 min. The products of error-prone PCR are referred to as the mutant insert in this text. The DsRed2 gene without mutations (Fig. 1 A) was amplified using the same primers and high-fidelity polymerase (TAKARA LA Taq DNA, Clontech Cat. No. RR002A) as a control for the procedure (referred to as the control insert). The PCR conditions for this were 94 °C for 2 min, followed by 30 cycles of 94 °C for 15 s, 60 °C for 30 s, 72 °C for 2 min, and a final cycle at 72 °C for 5 min. After PCR, the amplicons were verified on 1% agarose gel electrophoresis and purified using the Illustra GFX® PCR DNA and Gel Band Purification Kit (GE Healthcare).
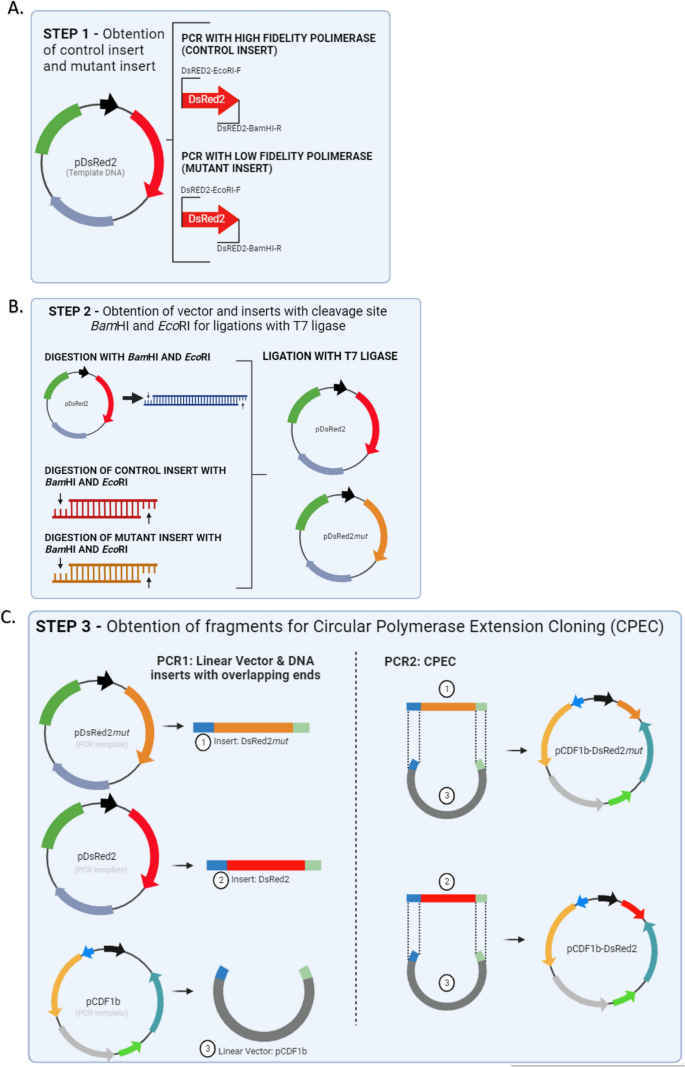
Graphic representation of the main methodological steps for comparing the Ligation-Dependent Process Cloning method (LDPC) and Circular Polymerase Extension Cloning (CPEC). ( A ) Step 1 – Obtaining the Mutant Insert by Error-Prone PCR and the Control Insert. The DsRed2 gene was isolated from the plasmid pDsRed2 through error-prone PCR using specific primers and conditions, resulting in the mutant insert. The control insert was also isolated from the plasmid pDsRed2, but a high-fidelity polymerase was used. ( B ) Step 2—Ligation-dependent process cloning. A vector was prepared by cleaving the pDsRed2 plasmid with BamHI-HF and EcoRI-HF enzymes, followed by digestion of all fragments (control insert and mutant insert from Step 1) using the same restriction enzymes, and ligation reactions were performed using T7 ligase. ( C ) Step 3—Circular Polymerase Extension Cloning – CPEC. The mutant insert, along with the control, was amplified via PCR, quantified, and cloned into the pCDF1b (GenBank Accession Number OR900361.1) expression vector using CPEC with overlapping primers.
Step 2—Ligation-dependent process cloning
Initially, the pDsRed2 plasmid was cleaved (Fig. 1 B) using the enzymes BamHI-HF (New England Biolabs, Cat. No. R3136) and EcoRI-HF (New England Biolabs, Cat. No. R3101) to get the vector. Afterwards, BamHI-HF and EcoRI-HF restriction enzymes were used to digest all fragments (control insert and mutant insert – Step 1). This digestion took place over an incubation time of 2 h at a temperature of 37 °C. The enzymes were inactivated for 20 min at 65 °C. Digested fragments were quantified on the Qubit fluorimeter (Life Technologies, Brazil) using the Quant-iT dsDNA BR Assay kit (Invitrogen, Brazil). A 1:1 ratio was used for the ligation reactions. The vector (pDsRed2) was at a concentration of 81.7 ng/µL and the inserts were at a concentration of 84.1 ng/µL. The ligation using the T7 ligase (New Englands, Biolabs Cat. No M0318) was carried out according to the manufacturer’s protocol and was conducted in triplicate.
Bacterial transformation for T7 ligation products
A total of the 1 µL of product from each ligation was transformed into 40 µL of electrocompetent Escherichia coli TOP 10 bacteria (0.2 cm cuvette, 2.5 kV/cm, 25 µF, 200 Ω, 1 pulse) using the Gene Pulser Xcell™ electroporation system (BioRad). The cells were grown in 480 µL of SOC medium (2% tryptone, 0.5% yeast extract, 0.05% NaCl, 2.5 mM KCl, 20 mM glucose) for 1:30 h at 37 °C with constant shaking at 243 g in a Stuart Shaking incubator SI500 orbital shaker (Stuart, Brazil). After incubation, the inoculants were seeded in plates containing Luria Bertani (LB) agar medium and antibiotic spectinomycin (100 µg/mL) and incubated for 16 h at 37 °C. The bacteria transformed with the product of each ligation were screened for strong fluorescence using the Safe Imager™ 2.0 Blue Light Transilluminator (Invitrogen) with excitation at 470 nm. The plates obtained were photographed and the total number of colonies on each plate was determined. The plates for the controls and mutants were quantified using microscopy and counted with ImageJ software.
Step 3—Circular polymerase extension cloning—CPEC
We utilized the construct that was obtained and chosen from Step 2 (pDsRed mut ) as a template for the construct that included the mutant insert. PCR reaction was performed using the primers Mut/Dsred2-F and Mut/Dsred2-R (Table 1 ) and the TAKARA LA taq high fidelity DNA polymerase (5U/µL TAKARA LA Taq, 10X LA PCR buffer II (Mg 2+ free, 25 mM MgCl 2 , 0.25 mM dNTP). The PCR conditions were one cycle of 94 °C for 2 min (initial denaturation) followed by 30 cycles of 94 °C for 15 s, 66 °C for 30 s, and 68 °C for 3 min, and a final elongation of 72 °C for 10 min. After PCR, the fragments (hereafter mutant) were quantified using the Quant-iT dsDNA HS Assay kit (Invitrogen, Brasil). The same procedure was done in the DsRed2 gene as a control. The mutant gene and the control were cloned into the pCDF1b expression vector (Novagen, Cat. No. 71330-3) (Fig. 1 C). The ligation of fragments (DsRed2 and DsRed mut ) with vector (pCDF1b) was done via CPEC with the primers PCDF-F and PCDF-R (Table 1 ). These oligonucleotides have an overlapping sequence (bases under-arrayed in the sequence) with the product mutant for CPEC to occur.
The PCR for CPEC was carried out using the TAKARA LA Taq enzyme (Clontech Cat. No. RR002A), following the conditions: 94 °C/2 min, 30 cycles of 94 °C/15 s, 63 °C/30 s, 68 °C/4 min and 1 final cycle 72 °C/5 min. The template DNA for the CPEC reaction was the double-stranded fragments of the mutant and the vector pCDF1b was added in a 1:1 ratio. In the first PCR cycle, the fragments are denatured. In the following cycles, the single strands are ringed in the sequence in which they overlap, and it is from this overlap that the fragments extend to form the double strand of the circular plasmid pCDF1b-DsRed2 mut and pCDF1b-DsRed2, respectively. The fragments were analyzed using 1% agarose gel electrophoresis.
Bacterial transformation for CPEC products
The expression vectors produced (pCDF1b-Mutant and pCDF1b-DsRed2) were transformed into electrocompetent Escherichia coli BL21-DE3 by electroporation (0.2 cm cuvette, 2.5 kV/cm, 25 μF, 200 Ω, 1 pulse) using the Gene Pulser Xcell™ electroporation system (BioRad). The transformed bacteria were seeded in plates containing Luria Bertani (LB) agar medium and antibiotic spectinomycin (100 μg/mL) and incubated for 16 h at 37 °C. After transformation, bacterial colonies were inoculated into liquid Luria Bertani (LB) medium, using antibiotic spectinomycin (100 μg/mL) as a selective agent, incubated for 16 h at 37 °C with constant shaking at 243 g in a Stuart Shaking incubator SI500 (Stuart, Brazil). Subsequently, the plasmids were purified using the Ilustra™- Plasmid Prep Mini Spin Kit (GE Healthcare). The plates obtained were photographed and the total number of colonies on each plate was determined. The plates for the controls and mutants were quantified using microscopy and counted with ImageJ software.
The selected bacterial colonies were inoculated into liquid Luria Bertani (LB) medium, using the antibiotic spectinomycin (100 μg/mL) as a selective agent, incubated for 16 h at 37 °C with constant shaking at 243 g in a Stuart Shaking incubator SI500 orbital shaker (Stuart, Brazil). Subsequently, the plasmids with mutant and control inserts were purified using the Ilustra™- Plasmid Prep Mini Spin Kit (GE Healthcare). After purification, the plasmids were sequenced using the oligonucleotides PCDFBGL-Seq-F and PCDFBGL-Seq-R (Table 1 ) to confirm binding using the CPEC and LDCP methodologies.
Statistical analysis
One-way ANOVA was used for the statistical analysis of the data, with a significance threshold of p < 0.05. To make sure the test assumptions were met, tests for homogeneity of variances and residuals’ normality were performed before to the ANOVA. Specifically, Levene’s test was used to assess the homogeneity of variances, and the Shapiro–Wilk test was employed to evaluate the normality of residuals.
Results and discussion
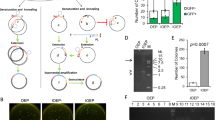
In order to enhance the efficiency of mutant gene library production, we compared two techniques for linking randomly mutated fragments (generated through epPCR) to the cloning vector. Initially, we employed the gene encoding the red fluorescent protein as a template for random mutagenesis through epPCR. Subsequently, the products epPCR-generated were linked to the cloning vector using two distinct methodologies: the cut-and-paste method, also known as ligation-dependent process cloning (LDCP), and the circular polymerase extension cloning (CPEC) method 10 , 12 . Both methodologies demonstrated efficiency, with colonies observed on all plates (Fig. 2 ).
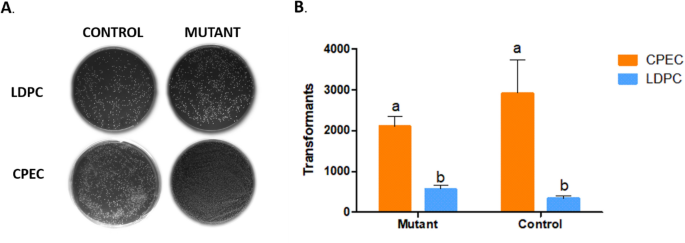
Comparison of colony numbers in Ligation Methods. ( A ) Photos of plates containing the colonies transformed with the vectors pCDF1b-Mutant (Mutant) and pCDF1b-DsRed2 (Control) using the Ligation-Dependent Process Cloning method (LDPC) and Circular Polymerase Extension Cloning (CPEC). ( B ) The average number of colonies obtained by comparing the ligation of the insert (mutant in orange bars and control in blue bars) with the vector (pCDF1b) using the LDPC and CPEC methods. The letters “a” and “b” denote statistically significant differences ( p < 0.005) in all cases, n = 3.
The CPEC-based ligation (Fig. 2 B) yielded a higher number of colonies (averaging 2106 colonies for mutant fragments and 2916 for control fragments) compared to the LDCP method (averaging 560 colonies for mutant fragments and 330 for control fragments). These results showed that ligation by CPEC was significantly more efficient than LDCP ( p < 0.05).
LDCP is a cloning technique that involves linking DNA fragments to the cloning vector through ligation-dependent processes. One advantage of LDCP is its relative simplicity, as it does not require many intricate steps or complex reagents. However, traditional restriction- and ligation-based cloning methods are often associated with low efficiency and time consumption. These methods can be complicated by factors such as incomplete restriction digestion, the effects of DNA methylation, and, at times, limited availability of suitable restriction endonuclease recognition sites. The traditional digestion-ligation method may work better in some circumstances. For instance, when a fragment needs to be released from a vector using restriction digestion and then subcloned into a different vector. But it’s important to remember that, in theory, DNA fragments can also be cloned using a variety of PCR-dependent methods, such as TA cloning 13 , 14 , Gateway cloning 15 , Gibson assembly cloning 16 , and CPEC 10 , 11 .
We choose CPEC because it is directional, positional, sequence-independent, and requires only two steps and one enzyme. To do this, we linearize the vector using PCR and create an insert that is complementary to the vector by adding 20 bp (Fig. 1 C). In this method, both the linear vector and the insert possess overlapping regions at their respective ends. Following denaturation and annealing, these complementary segments come together to form a hybrid molecule, and the extension occurs as they act as templates for each other, resulting in the formation of a full circular structure with two nicks. This circular construct can then be transformed directly into bacteria without the need for additional purification steps, simplifying the process significantly 10 , 13 . It is important to note that the observed efficiency of transformation in electroporation in the two methods compared (LDCP and CPEC) may be influenced by the salt concentration in the buffers used 17 , 18 . Future comparative studies utilizing heat shock transformation, which is less sensitive to ionic strength, could help further validate the observed differences between LDCP and CPEC.
In our study, CPEC not only confirmed the simplicity of the method but also demonstrated its efficiency, thereby enhancing the acquisition of gene libraries. The more clones we obtain, the higher the chance of finding the one with the desired phenotypic trait. The search for mutant genes with new phenotypic characteristics is an approach frequently employed in functional genetics and genetic engineering studies to understand how genes influence organism phenotypes and to develop desired traits in target organisms. The choice between CPEC and LDCP depends on the specific needs of the project and the convenience of the technique in each context. Nonetheless, our preference for CPEC is based on its simplicity, efficiency, and suitability for our research objectives. The field of molecular biology continues to evolve, offering a range of cloning techniques, and researchers should always consider the most appropriate method for their specific applications.
The binding of variant fragments of the DsRed2 gene using the CPEC methodology is more efficient when employed in the generation of gene libraries mutated by epPCR. The enhanced efficiency of binding variant genes into an expression vector results in a greater number of genes with the potential to exhibit novel phenotypes.
Data availability
The data and materials used in this study are available upon request from the corresponding author, D.V.A. The sequence data used in this study are available at https://www.ncbi.nlm.nih.gov for pCDF1b (Accession Number OR900361.1) and at https://www.uniprot.org for the plasmid pDsRed2 (Accession Number Q9U6Y8).
Puetz, J. & Wurm, F. M. Recombinant proteins for industrial versus pharmaceutical purposes: A review of process and pricing. Processes 7 , 476. https://doi.org/10.3390/pr7080476 (2019).
Article CAS Google Scholar
Wang, L. et al. Therapeutic peptides: Current applications and future directions. Sig. Transduct. Target. Ther. 7 , 48. https://doi.org/10.1038/s41392-022-00904-4 (2022).
Gardner, A. F. & Kelman, Z. DNA polymerases in biotechnology. Front. Microbiol. 5 , 659. https://doi.org/10.3389/fmicb.2014.00659 (2014).
Article PubMed PubMed Central Google Scholar
Labrou, N. E. Random mutagenesis methods for in vitro directed enzyme evolution. Curr. Protein Pept. Sci. 11 (1), 91–100. https://doi.org/10.2174/138920310790274617 (2010).
Article CAS PubMed Google Scholar
Leung, D. W., Chen, E. & Goeddel, D. V. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique 1 , 11–15 (1989).
Google Scholar
Bryksin, A. V. & Matsumura, I. Overlap extension PCR cloning: A simple and reliable way to create recombinant plasmids. Biotechniques. 48 (6), 463–465. https://doi.org/10.2144/000113418 (2010).
Article CAS PubMed PubMed Central Google Scholar
Geiser, M., Cébe, R., Drewello, D. & Schmitz, R. Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. Biotechniques 31 (1), 88–92. https://doi.org/10.2144/01311st05 (2001).
Tillett, D. & Neilan, B. A. Enzyme-free cloning: A rapid method to clone PCR products independent of vector restriction enzyme sites. Nucleic Acids Res. 27 (19), e26–e28. https://doi.org/10.1093/nar/27.19.e26 (1999).
Tseng, H. DNA cloning without restriction enzyme and ligase. Biotechniques 27 (6), 1240–1244. https://doi.org/10.2144/99276rr02 (1999).
Quan, J. & Tian, J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS ONE 4 (7), e6441. https://doi.org/10.1371/journal.pone.0006441 (2009).
Article CAS PubMed PubMed Central ADS Google Scholar
Quan, J. & Tian, J. Circular polymerase extension cloning. In DNA Cloning and Assembly Methods (eds Valla, S. & Lale, R.) 103–117 (Humana Press, Totowa, 2014). https://doi.org/10.1007/978-1-62703-764-8_8 .
Chapter Google Scholar
Quan, J. & Tian, J. Circular polymerase extension cloning for high-throughput cloning of complex and combinatorial DNA libraries. Nat. Protoc. 6 , 242–251. https://doi.org/10.1038/nprot.2010.181 (2011).
Clark, J. M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 16 (20), 9677–9686. https://doi.org/10.1093/nar/16.20.9677 (1988).
Zhou, M. Y. & Gomez-Sanchez, C. E. Universal TA cloning. Curr. Issues Mol. Biol. 2 (1), 1–7 (2000).
CAS PubMed Google Scholar
Katzen, F. Gateway(®) recombinational cloning: A biological operating system. Expert Opin. Drug Discov. 2 (4), 571–589. https://doi.org/10.1517/17460441.2.4.571 (2010).
Article Google Scholar
Gibson, D. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6 , 343–345. https://doi.org/10.1038/nmeth.1318 (2009).
Lee, M. J. et al. Optimal salt concentration of vehicle for plasmid DNA enhances gene transfer mediated by electroporation. Exp. Mol. Med. 34 (4), 265–272. https://doi.org/10.1038/emm.2002.37 (2002).
Rahimzadeh, M., Sadeghizadeh, M., Najafi, F., Arab, S. & Mobasheri, H. Impact of heat shock step on bacterial transformation efficiency. Mol. Biol. Res. Commun. 5 (4), 257 (2016).
CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
We would like to express our gratitude to the Molecular Biology Laboratory at the Federal University of Rio Grande (FURG) for their assistance, resources, and expertise, which were instrumental in the successful completion of our research. This study was funded by National Council for Scientific and Technological Development (MCTI/CNPQ Call No. 454689/2014-4) and by Coordination for the Improvement of Higher Education Personnel (CAPES), which provided the master’s scholarship.
Author information
Authors and affiliations.
Departamento de Biología, Universidad del Valle (UV), Cali, Colombia
Natalia Ossa-Hernández
Laboratório de Biologia Molecular, Instituto de Ciências Biológicas, Universidade Federal do Rio Grande (FURG), Rio Grande, Brazil
Luis Fernando Marins
Instituto de Biologia, Universidade Federal de Pelotas - UFPEL, Campus Universitário Capão do Leão s/n, Pelotas, RS, 96160-000, Brazil
Daniela Volcan Almeida
You can also search for this author in PubMed Google Scholar
Contributions
All authors (N.O.-H., L.F.M. and D.V.A.) were involved in the drafting and critical review of the manuscript and all contributed to the final writing. Experimental assays, results analysis, and writing were carried out by N.O.-H and D.V.A.
Corresponding authors
Correspondence to Natalia Ossa-Hernández or Daniela Volcan Almeida .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ossa-Hernández, N., Marins, L.F. & Almeida, D.V. Combination of error-prone PCR (epPCR) and Circular Polymerase Extension Cloning (CPEC) for improving the coverage of random mutagenesis libraries. Sci Rep 14 , 15874 (2024). https://doi.org/10.1038/s41598-024-66584-y
Download citation
Received : 19 February 2024
Accepted : 02 July 2024
Published : 10 July 2024
DOI : https://doi.org/10.1038/s41598-024-66584-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- DNA recombination
- Gene amplification
- Molecular cloning
- Random mutations
- Variant genes
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

COMMENTS
Ligation Calculator. This tool will calculate the mass of insert required at several molar insert:vector ratios in the range needed for typical ligation reactions. ... x ratio of insert to vector lengths. Videos. NEBioCalculator - using the ligation module Unable to load all required components properly. Please try reloading the page. If that ...
DNA. Heat inactivate (Antarctic Phosphatase, Quick CiP, rSAP) before ligation. Keep total DNA concentration between 1-10 µg/ml. Vector: Insert molar ratios between 1:1 and 1:10 are optimal for single insertions (up to 1:20 for short adaptors). Insert: vector molar ratio should be 6:1 to promote multiple inserts.
The volume of vector DNA and insert DNA used in the ligation will vary depending on the size of each and their concentration. However, for most standard cloning (where the insert is smaller than the vector) a 3 insert : 1 vector molar ratio will work just fine. We recommend around 100ng of total DNA in a standard ligation reaction. Use a (Link ...
Insert amount (ng) = Vector amount x Insert size / Vector size x (Molar ratio) Molar ratios (Insert/Vector ratio) of 3:1 are used for optimizing cohesive end ligations whilst higher molar ratios are used for blunt end ligations. Ligation is essential in DNA replication in the human body whilst exogen ligation reactions are used to create ...
To determine the best ratio of insert:vector to use for cloning, you may have to try different ratios ranging from 1:1 to 15:1, but a 3:1 ratio is a good place to start. For blunt-end ligation, be sure to adjust the insert:vector ratio and increase to 10:1 to optimize your result.
Ligation Calculator. This tool determines the mass of insert needed for various inserts:vector molar ratios within the range suitable for typical ligation reactions. Required insert DNA mass [g] = vector DNA mass [g] x {insert DNA length / vector DNA length} x desired insert to vector molar ratio. Notes: Desired ratio is one from set {1, 2, 3 ...
We typically recommend starting with a 1:3 vector-to-insert molar ratio for an initial ligation, although this depends on the length of your vector and insert fragments. If the size of the vector and insert are very similar, then a 1:1 ratio is the best place to start. As the size of the insert gets smaller in relation to the vector, then the ...
Ligation of Vector and Insert. Use a molar ratio between 1:1 and 1:10 of vector to insert (1:3 is typical). Use NEBioCalculator to calculate molar ratios. If using T4 DNA Ligase (NEB # M0202) or the Quick Ligation™ Kit , thaw and resuspend the Ligase Buffer at room temperature. If using Ligase Master Mixes, no thawing is necessary.
LIGATIONS. Vector:Insert Ratio. After the vector and insert DNA have been prepared for ligation, determine the concentration either by spectrophotometer or by agarose gel against a known amount of standard marker. Test various vector:insert DNA ratios in order to find the optimum ratio for a particular vector and insert.
Hence, higher chance of proper ligation. Thus vector to insert ratio is ideally 1:3. Depending on the requirement, it can be changed to 1:5 or even 1:7 to increase chances of getting positive ...
The vector:insert ratio changes, depending on the insert, even if you use the same vector. If you use the same vector:insert ratio for many different inserts and the insert size increases or decreases, recalculate the amount of insert needed for ligation using the equation above or our handy BioMath Calculator to ensure the molar ratio stays ...
LIGATIONS Vector:Insert Ratio After the vector and insert DNA have been prepared for ligation, determine the concentration either by spectrophotometer or by agarose gel against a known amount of standard marker. Test various vector:insert DNA ratios in order to find the optimum ratio for a particular vector and insert.
Ligation Calculator. This tool will calculate the mass of insert required at several molar insert:vector ratios in the range needed for typical ligation reactions. Choose a DNA, RNA, qPCR calculator from NEB, a leader in production and supply of reagents for the life science industry.
insert and vector to use for the ligation reaction. The volume of vector DNA and insert DNA used in the ligation will vary depending on the size of each and their concentration. However, for most standard cloning (where the insert is smaller than the vector) a 3 insert: 1 vector molar ratio will work just fine.
If your insert is smaller than the vector, say if you're trying to ligate a 1kb insert into a 3kb vector, you'll need a higher ratio, in this case about a 3:1 molar ratio of insert to vector. If your inserts are very small, even higher ratios may be needed, sometimes as high as 20:1. You can calculate the amount of DNA you need for your ...
A molar ratio between insert and vector should be calculated. Typically, the insert/vector ratio for sticky-end ligations ranges between 1:1 and 3:1. For blunt-ended ligations, the insert/vector ratio should be at least 10:1. The number of moles for vector and insert can be calculated as follows: (g DNA)-----
DNA calculations to convert µg to pmol for double-stranded and single-stranded DNA, convert micrograms of DNA to pmol ends, calculate vector:insert molar ratio and convert OD260 readings to µg/ml. Also calculate molarity of solutions, perform molar conversions, calculate dilutions and perform other calculations common in molecular biology labs.
Linear vector DNA 20-100 ng Insert DNA 1:1 to 5:1 molar ratio over vector Ligase buffer 2 μL 1 U Water, nuclease-free to 20 μL Total volume 20 μL • If more than 2 U of T4 DNA Ligase is used in 20 μL reaction mixture, it is necessary to purify DNA (by spin column or chloroform extraction) before electrotransformation. Blunt-end Ligation 1.
Keep in mind, the vector:insert ratio will change depending on the size of the insert. In most cases, a 1:1 or 1:3 molar ratio of vector:insert works well, but you may want to consider 1:5, 5:1 and even a 10:1 ratio. The equation for calculation of the amount of insert required at a specific molar ratio of vector:insert is: [(ng of vector × kb ...
My first ligation I did a insert to vector molar ratio of 4:1, after transformation into in-lab-made competent Top 10 E. Coli, I got 10 colonies on LB/Amp plates. I mini-prepped those 10 colonies ...
Hi, usually if you do a ligation it is recommended to use a vector- insert ratio 1:3 in general. I think this works great if the insert is smaller or maybe the same size as the vector. But what if the insert is about 3 times bigger than the vector. Is it then useful to try it the other way round: 3 times the vector to insert?
I'd guess that the exact ratio is less important than having excess insert comparative to the vector concentration. Having a ratio higher than 1:1 is usually ideal, as you have a better chance of successful ligation by increasing the chance of interaction between insert and vector; however it depends on the size of your insert/vector and the ...
A 1:1 ratio was used for the ligation reactions. The vector (pDsRed2) was at a concentration of 81.7 ng/µL and the inserts were at a concentration of 84.1 ng/µL. The ligation using the T7 ligase ...